Critical stereocontrol by inter-amino distance of supramolecular photocyclodimerization of 2-anthracenecarboxylate mediated by 6-(ω-aminoalkylamino)-γ-cyclodextrins
Abstract
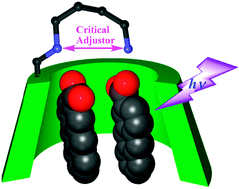
A series of 6-(ω-aminoalkylamino)-6-deoxy-γ-cyclodextrins (CDs) 5–8 with varying inter-

 Please wait while we load your content...
Please wait while we load your content...